
Events
-
Timofey Petrov appointed Director General of Innotech
25 October 2021

Russian pharmaceutical company NovaMedica announces appointment of Timofey PETROV as the head of the R&D Center NovaMedica Innotech (subsidiary of NovaMedica).
-
Innotech renews manufacturing license
21 September 2021
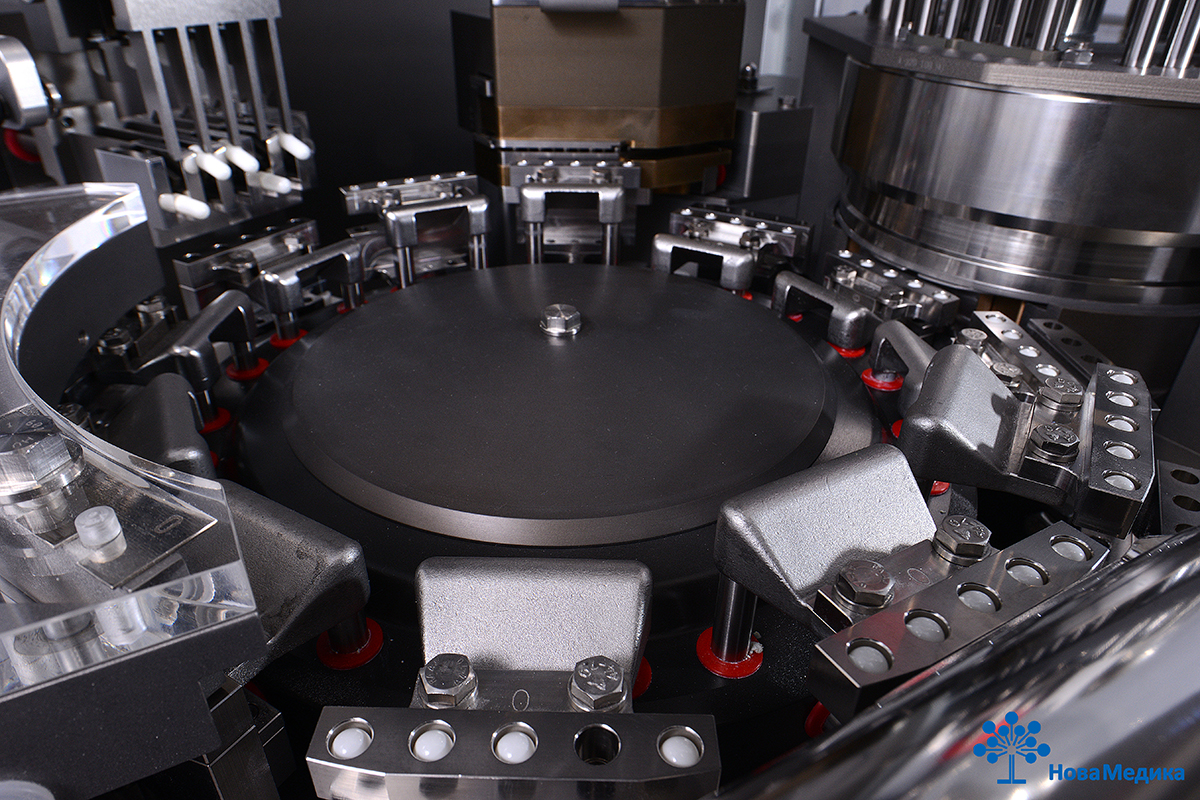
R&D Center NovaMedica Innotech has successfully passed the audit of the Ministry of Industry and Trade and received a renewed and extended manufacturing license for production of pharmaceutical drugs, which creates new contracting opportunities and, in addition to the already existing services, allows carrying out primary and secondary packaging of biological products.
-
R&D Center NovaMedica Innotech joined Avifavir production efforts in Russia
04 March 2021
R&D Center NovaMedica Innotech (subsidiary of the pharmaceutical company NovaMedica, investment project of RUSNANO) announced release of the first commercial batch of the anti-COVID drug Avifavir® under the partnership with Kromis LLC, joint venture of the Russian Direct Investment Fund (RDIF) and the company ChemRar. NovaMedica Innotech will manufacture 100,000 units of Avifavir per month.
-
03 March 2021

We have already made a tradition of guided tours to the R&D Center NovaMedica Innotech for our most curious and precious visitors – children who come to see what their mothers and fathers do at work.
-
PharmNews: NovaMedica Innotech debuts on CPhI
09 October 2018

The international exhibition CPhI has started in Madrid. And today NovaMedica Innotech is in our focus as the company that has made a bright debut at the exhibition. This Technological Center has launched in April 2017 and Moscow Mayor Sergei Sobyanin attended the opening ceremony.
-
How Can the Russian R&D Industry Help the Global One?
03 October 2018
The current model of the pharmaceutical business in the context of R&D in Russia reflects the world trends. One of the striking examples is R&D Center of NovaMedica Innotech. It belongs to the group of CDMO (contract development and manufacturing organizations), whose task is to provide services following the 'one contact’ principle: pharmaceutical development, technology development, technology transfer, and manufacturing of small batches of products - both for clinical trials and for manufacturing of finished products batches (based on the GMP license).
-
Penetrating the European Market
02 October 2018

NovaMedica Innotech is a first-timer at Cphl WorldWide. This is the first opportunity for the company to introduce its products to the European market. NovaMedica Innotech Technology Centre opened its doors in April 2017 in Moscow. It possesses a unique range of innovative Technology platforms for the development and pilot productions of advanced, completive and effective pharmaceuticals.
-
07 June 2018

The Russian pharmaceutical company NovaMedica (RUSNANO's portfolio company) has registered a unique drug Fissario® for proctologic diseases treatment, based on a new mechanism of action not used in products currently on the market. This is the first drug developed within own R&D program of NovaMedica. It is planned that it will be available to patients already in 2019.
-
07 February 2018

Russian pharmaceutical company NovaMedica (investment project of Rusnano) announces start of its cooperation in the area of transfer of knowledge and technologies between the R&D Center of NovaMedica Innotech and the German company Evonik – one of the leading companies in the international market of excipients manufacturing for the pharmaceutical industry. This partnership will result into creation of a training facility for transfer of competences in the area of pharmaceutical technologies at the premises of NovaMedica Innotech in Russia.
-
26 December 2017

Russian pharmaceutical company NovaMedica, investment project of Rusnano, informed about receipt of conclusion of the Ministry of Industry and Trade of the Russian Federation over compliance of the facility of the R&D Center of NovaMedica Innotech (subsidiary of NovaMedica) with the Russian standards of the Good Manufacturing Practice (GMP). The certificate issued basing on the integrated audit of the MIT RF commission, proves that the processes of development, manufacturing and quality control at NovaMedica Innotech are organized in accordance with the high standards of quality and manufacturing of tablets, soft and hard gelatin capsules and pellets at its manufacturing facilities meet the Russian GMP requirements.
Our news
-
05 November 2024
-
03 November 2024
-
October 10 – World Mental Health Day
10 October 2024
Media Center
-
Siberian State Medical University and Skoltech to start production of bone tissue fillers
22 November 2024
-
Sechenov University is creating a digital drug reference book
21 November 2024
-
Skills shortage still holding back pharma's digital transformation: survey
21 November 2024
-
Trials for Russia’s first CAR-T drug “Utzhefra” have started
20 November 2024