
The Way We're Testing Antibiotics Is All Wrong
15 October 2015
There's a big problem in the fight against deadly superbugs.
For 50 years, hospitals have used a single test to decide how to treat the most stubborn infections. But according to a growing body of research, that test is now wrong more often than we'd thought. All because of the rise of antibiotic-resistant bacteria that behave one way in lab tests and another way in the human body.
The findings have huge implications for how doctors fight the growing problem of so-called superbugs, which can't be easily treated with antibiotics. The bacteria infect 2 million people each year in the U.S. alone, and kill 23,000, according to the Centers for Disease Control.
“We’re saying the standard way the world does this is wrong,” said Michael J. Mahan, a professor of microbiology at the University of California, Santa Barbara. That standard protocol, established in the 1960s, is called antibiotic susceptibility testing: Bacteria are grown in a solution called Mueller-Hinton broth, and then attacked with various antibiotics to see which one works best. Doctors around the world use the test to decide which antibiotic to use for which bacterial infection.
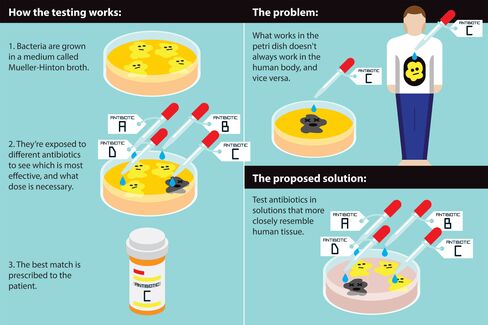
But Mahan in August published an article in the journal EBioMedicine that shows blind spots in the protocol, which hasn't changed much since it was instituted as the gold standard across medical labs several decades ago.
When his team tested salmonella in the petri dishes that labs typically use, an antibiotic called polymyxin killed the bacteria. But when they grew salmonella in petri dishes formulated with a material that more closely resembles the cells the bacteria infect, the antibiotic was useless. They concluded that the bacteria's defenses—essentially the mechanisms that make superbugs "super"—can switch on or off depending on their surroundings. The salmonella had turned on its superpowers.
Another recent study led by Victor Nizet, a professor of pediatrics and pharmacy at UC San Diego, showed a similar result. An antibiotic called azithromycin, which fails to stop antibiotic-resistant strains of certain bacteria in lab tests, nonetheless worked when tested in mice. In Nizet's experiment, what appeared to be a "superbug" in standard tests lost its superpowers in animal tissue. These tests have "nothing to do with the patient,” Nizet said. "The patient’s not made out of Mueller-Hinton broth."
"It’s really clear from these papers that there are key examples where the way we’ve been doing things up until now is probably inadequate," said Stanley Maloy, dean of the college of sciences at San Diego State and past president of the American Society for Microbiology, who wasn't involved in the research. "It is a very big shift."
The antibiotic susceptibility test is also how pharmaceutical companies evaluate the effectiveness of potential new drugs. That means molecules that drugmakers have discarded as ineffective may in fact be potential cures. And the world urgently needs new drugs: The most recent class of antibiotics was developed in the 1980s, according to a World Health Organization report last year, which warned that bacteria are adapting to drugs faster than we're creating new ones to fight them.
Antibiotics remain effective against most bacteria, and the standard lab test is usually pretty good at deciding what drug to use, said Dan Andersson, a professor of medical bacteriology at Uppsala University in Sweden. "As a rule, they work surprisingly well,” he said. But the research from Mahan and Nizet could change how doctors approach hard-to-treat infections. "There are certain types of resistance where this may be very important," Andersson said.
Mahan and Nizet haven't tested their findings in human patients yet. But what they've identified is consistent with anecdotal reports from doctors: Sometimes a lab test wrongly predicts that a certain drug will work against an infection, and switching to a different medication is more effective. Mahan said his findings have immediate implications for doctors who encounter stubborn infections: "If the drug doesn’t work, switch the drug."
Right now hospitals don't perform the kinds of tests Mahan and Nizet used to detect when pathogens switch their resistance superpowers on and off. Mahan said labs could easily expand their protocol to test difficult-to-treat bacteria in the kind of media he used, in addition to the standard Mueller-Hinton broth. Andersson is more skeptical. "There are many things that clinical microbiology labs in principle could do, but they don’t do it because it’s too complicated and too expensive," he said.
Nizet said it’s time to rethink how we pick antibiotics and vet new drugs. He envisions a personalized approach that could measure antibiotic effectiveness in a lab test using the patient's own cells. "If that was me, and I had this drug-resistant infection, that’s kind of what I’d like to know: Why do the testing in something that has nothing to do with my body?"
PrintOur news
-
14 March 2024
-
26 February 2024
-
NovaMedica team wishes you a Merry Christmas and a Happy New Year!
26 December 2023
Media Center
-
Research calls for greater investment in Alzheimer’s clinical trials
03 May 2024
-
Stress impact on protein particle formation for monoclonal antibody formulation
03 May 2024
-
Health Ministry registered Russian drug for ankylosing spondylitis
02 May 2024
-
Production of finished drugs in Russia grew by 13.8%
02 May 2024