
It pays to seek EMA advice, analysis shows; the earlier, the better
24 April 2015
LONDON – Taking the EMA's advice on trial design is both voluntary and costly, but a new analysis shows that doing so vastly increases the likelihood of getting marketing approval.
A full 67 percent of clinical development plans submitted for scientific advice were not good enough, with proposed trial designs that would fail to generate adequate data on which to assess the benefit/risk of products.
Companies that changed their development plans in line with feedback from the EMA's scientific advice working party were more likely to be granted a marketing authorization, according to research by EMA staff, which is due to be published in Nature Reviews Drug Discovery in May.
The researchers had previously shown that compliance with EMA scientific advice on clinical trial design correlates with success in obtaining marketing approvals. The latest research aimed to show cause and effect.
Researchers analyzed 118 marketing authorization applications (MAAs) received between 2008 – 2012 for products for which scientific advice had been sought, to see if sponsors changed trial designs as a result and whether that affected the outcome.
Companies that took advice opted for early interaction, with 76 percent asking for feedback before the start of pivotal trials. That also translated through to a better chance of eventual success, with 78 percent of products getting approval, compared to 64 percent of products where scientific advice was received once a pivotal trial was in progress. (See Figure 1, below.)
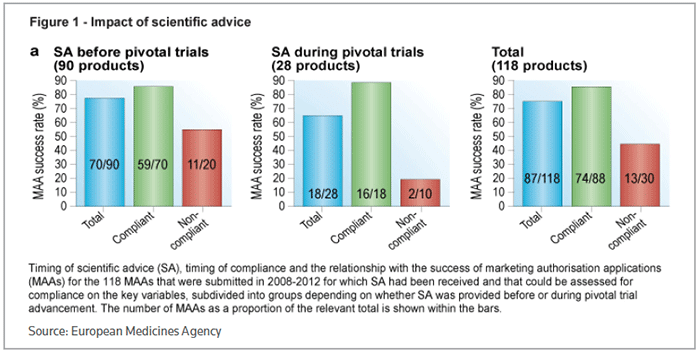
The level of compliance with scientific advice was assessed on three parameters: the choice of primary efficacy endpoint, the choice of comparator treatment and the statistical methodology. Overall, clinical development programs that complied with scientific advice had an 84 percent success rate in securing approval for the product under test, compared to 43 percent for noncompliant programs.
Of the 67 percent of clinical development plans that were initially deemed inadequate, 63 percent of plans (50 products) were modified in the light of the EMA's scientific advice. Of those 50 products, 43 went on to get marketing approval.
Of the 37 percent (29 products) for which the development plans were assessed as inadequate, but which were not amended in line with the EMA's scientific advice, 12 were approved and 17 received a negative opinion.
The researchers also investigated whether accepting scientific advice had any impact on the actual assessment of an MAA by the EMA's Committee for Medicinal Products for Human Use (CHMP). Here they found compliance was associated with a reduction in major objections raised and a review that was, on average, 61 days shorter.
There is "a growing demand for interaction with regulators prior to marketing authorization submission," according to the researchers. The EMA began offering scientific advice in 1996. By 2012 the proportion of products at MAA-stage that had received scientific advice reached 70 percent, up from 47 percent five years earlier in 2007.
Scientific advice does not come cheap, with a basic fee in 2015 of €83,500 (US$96,000). Nor can companies expect a comprehensive appraisal: The agency is only prepared to comment on development strategies. It gives the scientific advice in the form of responses to questions, and it is not unusual to hear companies complain about the difficulties of knowing what to ask.
There is a lower fee for companies that are classed as SMEs and for advanced therapies based on cells, genes or human tissue. There is no fee for advice on developing pediatric products.
The researchers also pointed out that the CHMP is not bound by the scientific advice and that is reflected in the fact that not all development programs that comply are successful, and vice versa.
PrintOur news
-
14 March 2024
-
26 February 2024
-
NovaMedica team wishes you a Merry Christmas and a Happy New Year!
26 December 2023
Media Center
-
Research calls for greater investment in Alzheimer’s clinical trials
03 May 2024
-
Stress impact on protein particle formation for monoclonal antibody formulation
03 May 2024
-
Health Ministry registered Russian drug for ankylosing spondylitis
02 May 2024
-
Production of finished drugs in Russia grew by 13.8%
02 May 2024