
Innotech renews manufacturing license
21 September 2021
 R&D Center NovaMedica Innotech has successfully passed the audit of the Ministry of Industry and Trade and received a renewed and extended manufacturing license for production of pharmaceutical drugs, which creates new contracting opportunities and, in addition to the already existing services, allows carrying out primary and secondary packaging of biological products.
R&D Center NovaMedica Innotech has successfully passed the audit of the Ministry of Industry and Trade and received a renewed and extended manufacturing license for production of pharmaceutical drugs, which creates new contracting opportunities and, in addition to the already existing services, allows carrying out primary and secondary packaging of biological products.

Natalia KOSAREVA, Director for Quality at Technological Center NovaMedica Innotech:
“The Regulator gave a positive assessment to NovaMedica Innotech’s compliance with the strict GMP requirements. At the next stage, we intend to obtain an EAEU GMP Certificate, which will allow us to expand the geography of our clients within the boundaries of that region. Our partners can be sure that the services offered by NovaMedica Innotech meet the global standards. We timely do everything to ensure smooth transition to the harmonized EAEU requirements”.
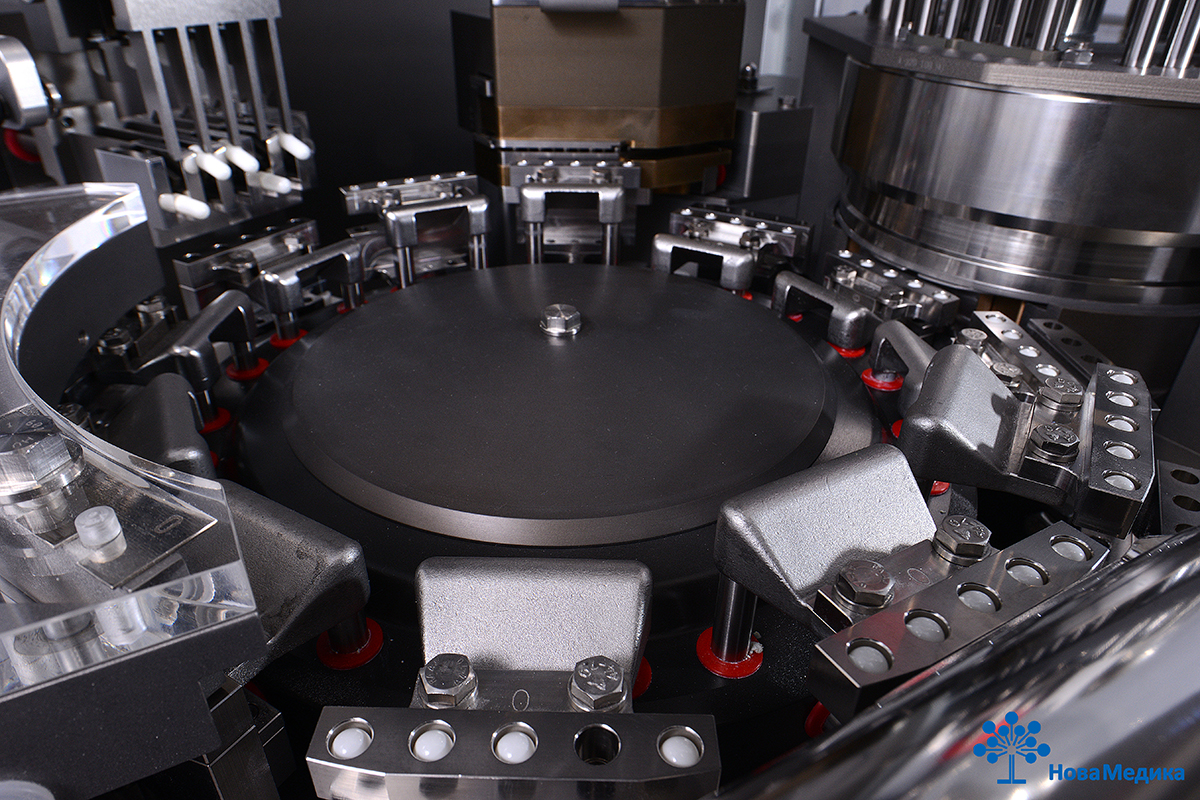
The manufacturing capacity of R&D Center NovaMedica Innotech is up to 120 mln pharmaceutical units per year (tablets, capsules, solid gelatin capsules with various fillers: micro-tablets, pellets, granules, powders). The manufacturing and laboratory capacities of the R&D Center allow scaling processes, carrying out technology transfers, producing samples for clinical trials and ensuring commercial release of solid dosage forms. Innotech’s manufacturing sites have been provided with advanced equipment that makes it possible to manufacture not only generics, but smart innovation products as well.
 |
 |
NovaMedica Innotech is a 100% subsidiary of NovaMedica, a project of Rusnano, a Skolkovo resident, a rapidly developing Russian pharmaceutical R&D center opened in April 2017 at Technopolis Moscow. NovaMedica Innotech specializes in development, introduction and manufacturing of innovative, competitive and effective drug products.
PrintOur news
Media Center
-
Vaccination against the plague using a Russian drug is currently being carried out in Mongolia
24 July 2024
-
24 July 2024
-
Russia will conduct an experiment to block the supply of low-quality products to hospitals
23 July 2024
-
With seventh person seemingly cured of HIV, signs of hope for a broader cure
23 July 2024