
Financial scorecard: biotech continues its great start
02 March 2015
As February moves into its final trading week the biotech sector continues to roll along, setting the pace for another strong year.
Following a volatile month for the capital markets in January, calmer heads have so far prevailed despite ongoing energy sector turbulence and financial uncertainties surrounding Greece and the potential ramifications for the rest of Europe. The Nasdaq Composite index has posted a more than 5 percent gain month to date and the Dow Jones Industrial Average performed almost as well, jumping 3.3 percent. The comparatively positive market conditions in the U.S. also helped the BioWorld Biotech Blue Chip index gain 3.7 percent in value. Year to date the index has been on a tear and is up 10 percent. (See BioWorld Blue Chip Index, below.)
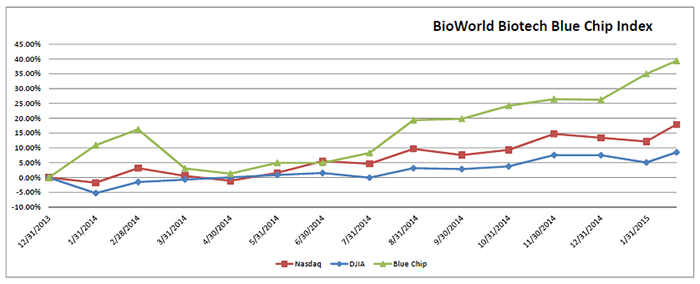
It hasn't been biotech's top two companies by market cap that have led the group's charge. In fact, the performances of Gilead Sciences Inc. and Amgen Inc. have been mixed so far this month, with their share values down 2 percent and up 3 percent, respectively.
For Amgen, the subject of biosimilars has given investors pause for thought. On the plus side, the company recently reported positive data from two phase III studies on ABP 501, which could become the first product from the expanding biosimilars program, for which the Thousand Oaks, Calif.-based firm is anticipated five commercial launches between 2017 and 2019. Results from the latest phase III study showed that ABP 501, given to patients with moderate to severe rheumatoid arthritis, met the primary endpoint, demonstrating clinical equivalence when compared to Humira (adalimumab, Abbvie Inc.), a monoclonal antibody designed to block TNF-alpha that is set to lose patent protection at the end of 2016 in the U.S., as assessed by ACR20 measurements (20 percent or greater improvement in ACR assessment) at week 24. (See BioWorld Today, Feb. 5, 2015.)
On the downside, the company reported in its fourth quarter financial results that despite higher than expected profits, bolstered by strong sales of Enbrel (etanercept), sales of its granulocyte-colony stimulating factor (G-CSF) used to treat neutropenia, Neupogen (filgrastim), was impacted by U.S. competition, inventory issues and foreign exchange rates and fell 17 percent year over year.
The results are an early indication of increasing competition from the likes of Teva Pharmaceutical Industries Ltd.'s unofficial biosimilar, Granix, with further potential erosion of Neupogen sales later this year if Sandoz Inc.'s biosimilar, Zarxio, gets the green light for marketing. It received a positive FDA panel vote last month. (See BioWorld Today, Jan. 8, 2015.)
Sales of Gilead's leading hepatitis C virus (HCV) drugs continued to boom in the fourth quarter despite new competition, totaling about $3.8 billion, and helped lead the company to a profit more than four times higher than the same quarter last year.
Competition from Abbvie's Viekira Pak (ombitasvir, paritaprevir, ritonavir and dasabuvir), approved in December for patients with chronic genotype 1 infection – which make up nearly three-quarters of Gilead's HCV patient base – has put added pressure on Gilead as it negotiates discounted pricing with pharmacy benefit managers, insurers and other payers. Gilead's shares (NASDAQ:GILD) closed Thursday at $102.73, down 2 percent so far in February.
One of the leading gainers was Shire plc, whose shares are up 8.4 percent this month. The company didn't have to wait long for a return on its $5.2 billion acquisition of NPS Pharmaceuticals Inc. The FDA approved NPS' Natpara, a recombinant human parathyroid hormone (PTH), as an adjunct to calcium and vitamin D to control hypocalcemia in patients with hypoparathyroidism. The rare endocrine disorder is characterized by insufficient levels of PTH. (See BioWorld Today, Jan. 27, 2015.)
Some of the cash expended in the acquisition was recouped from the receipt of the $1.635 billion break fee in relation to Abbvie's terminated offer for Shire. According to its latest financial results, the company closed 2014 with about $2.12 billion in cash.
GROWTH COMPANIES
Companies with market caps in the range of $1 billion to $3 billion comprising the BioWorld Biotech Growth Index kept pace with their larger counterparts, pushing up the index by 1.5 percent in February and almost 12 percent since the beginning of the year. (See BioWorld Biotech Growth Index, below.)
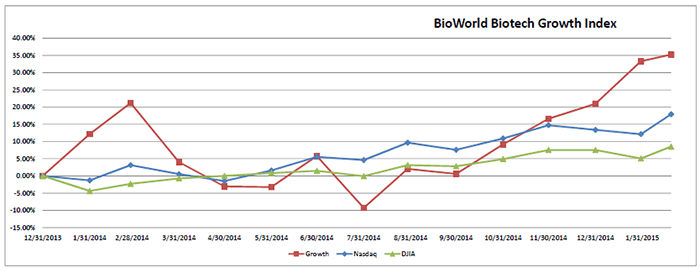
Public companies also have taken advantage of the positive environment to garner almost $6 billion from follow-on and other financings.
According to BioWorld Snapshots, 48 follow-on deals have been completed in 2015 so far at an average of $110 million per transaction.
Topping the deal scale was the $904 million underwritten public offering by Biomarin Pharmaceutical Inc. to help fund its recent acquisition of Prosensa Holding NV, of Leiden, the Netherlands. Cambridge, Mass.-based Alnylam Pharmaceuticals Inc. padded its bank balance to the tune of $450 million, pricing of an underwritten registered public offering of about 4.7 million shares of its common stock at $95 per share.
Last week, Neurocrine Biosciences Inc., a San Diego company developing drugs for neurological and endocrine disorders, banked $250 million in a public offering of about 6.9 million shares priced at $36 each to fund a growing roster of trials, rising R&D costs and plans to market its phase III tardive dyskinesia therapy, NBI-98854, ahead of an anticipated 2016 filing of its new drug application for the movement disorder. (SeeBioWorld Today, Feb. 20, 2015.)
PRIVATE HAUL
It also has been a good start to the year for private biotech companies, which have raised almost $1.5 billion. Interestingly, deal flow numbered 45 as compared to 47 at this stage last year, but those transactions generated $806 million.
The top deal by a wide margin so far is Cambridge, Mass.-based Moderna Therapeutics Inc.'s whopping $450 million financing that will fuel and expand its messenger RNA therapeutics platform across a number of therapeutic indications.
Given the present pace of transactions, the quarter is on pace to see a record $3 billion raised by private companies, almost three times the average amount normally raised in a quarter by biotech companies.
PrintOur news
-
06 September 2024
-
NovaMedica expands its line of products for the treatment of Alzheimer’s disease
22 August 2024
-
Children visited the NovaMedica Innotech pharmaceutical manufacturing facility
14 June 2024
Media Center
-
87% of cancer patients give positive ratings for medical care quality
18 September 2024
-
The Russian market of fillers and biorevitalizers grew by 21% in the first half of 2024
18 September 2024
-
Pharma’s Patient Reach Problem: Billions Still Left Behind
17 September 2024
-
17 September 2024