
Clinical trials in the New Health Economy: Digital tools and data driving awareness, engagement and retention
10 May 2018
New digital tools and services can help biopharmaceutical companies overcome the delays and bottlenecks endemic to drug research and development and can improve patient experiences and retention during clinical trials. Making trial participation more convenient and relevant to patients can help bring new drugs to market more rapidly and distinguish products in competitive therapeutic areas.
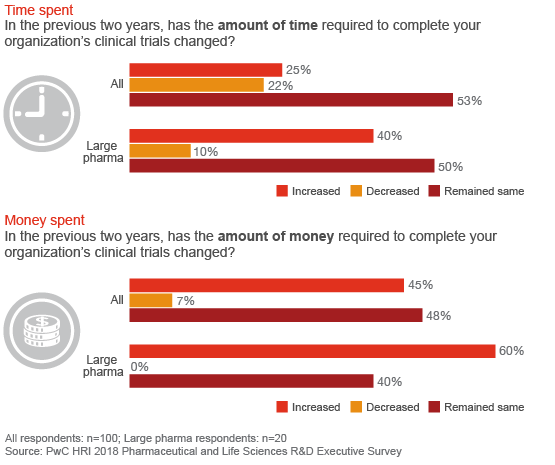
For most companies, the time and money needed to complete a clinical trial has remained the same or increased
The traditional process for conducting randomized, controlled clinical trials has been slow to fully harness new digital tools and plug into the massive amounts of consumer health data and information now available. New data sets collected by digital devices, online platforms and electronic health records are generating new insights that illuminate the nature and progression of disease and patients’ unmet needs. But the R&D enterprise continues to struggle with the same challenges it has faced for more than a decade.
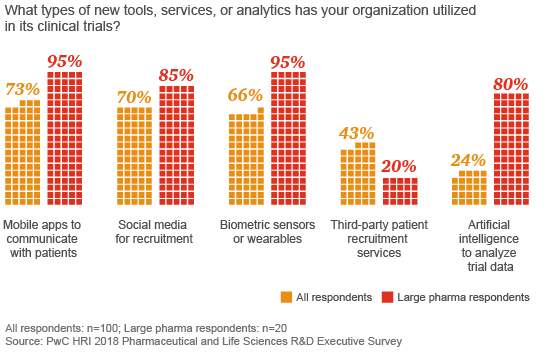
Larger companies have adopted new digital tools, such as wearables and artificial intelligence, more quickly than smaller companies
While the biopharma industry has begun to adopt new tools, such as artificial intelligence for data analyses and devices for digital data capture, most companies aren’t using technology to enable trial participation outside a testing facility, despite the cost savings, added convenience for patients and other potential advantages. Among R&D executives surveyed by HRI, only 8 percent had conducted a trial that included remote clinical data collection outside of a medical facility, although 30 percent of executives representing the largest biopharmaceutical companies reported using remote data collection.
Recommendations
Communicate early with regulators about trial design and protocols. Drug regulatory agencies in the US and Europe have expressed a willingness to work with trial sponsors to help design and enable remote data collection. Biopharmaceutical companies should take advantage of new opportunities to communicate—and collaborate—with regulators on trial protocols and digital data collection methodologies to ensure data integrity and successful trial execution. For example, electronic patient-reported outcomes (ePROs), particularly in oncology, are being tested in collaboration with the FDA regulators.
Create an intelligent data environment. Maintaining strong data integrity during clinical trials—regardless of where a trial takes place—is critical. Trial sponsors should review their data and analytics capabilities and decide whether to build them out further or partner with data or technology providers to create an enterprise capability that will support clinical trials across different R&D programs and therapeutic areas. Strong data capabilities may help enable novel trial designs or expedited review opportunities, or could be used to collect and analyze adverse events. Drugmakers also should use real-world evidence to support regulatory submissions, for additional drug indications, and to inform new drug development.
Build your own solution for more efficient trials. Numerous traditional healthcare companies, nonprofits and new entrants are bringing unique capabilities to the clinical trial process, to accelerate patient recruitment, data collection and analysis, and access to new therapies. Organizations that can identify bottlenecks in the trial process, such as trial recruitment, may be able to partner with technology providers, EHR vendors and other third parties to create an ecosystem that brings a best of breed approach to trial recruitment. Such a system would include patient education and advocacy as well as technological solutions.
Our news
-
14 March 2024
-
26 February 2024
-
NovaMedica team wishes you a Merry Christmas and a Happy New Year!
26 December 2023