
In biopharmaceutical research, if at first you don’t succeed, try again
26 August 2016
In case you missed it, an article in STAT News today profiles setbacks inside the process of researching new treatments and cures for patients. For America’s biopharmaceutical research companies, setbacks are far more common than success, but these stumbling blocks provide invaluable knowledge that help guide and direct researchers to get one step closer to the next scientific advancement.
Because of the complex nature of human diseases, the research and development (R&D) process is often arduous. In fact, it takes more than 10 years and $2 billion to develop a new medicine. While setbacks are an inherent part of this complex process, the odds of success are low – just 12 percent of drugs entering clinical trials ever make it to patients.
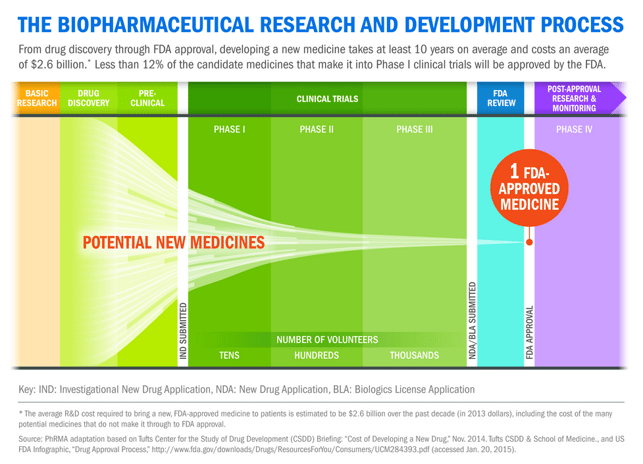
These challenges will only grow as biopharmaceutical researchers continue to focus on some of the most complex diseases and use completely new approaches to fight disease on the molecular and genetic level.
While the science to find new treatments and cures is only getting harder, America’s biopharmaceutical researchers and scientists are tireless in their pursuit of new treatments and cures for patients. With over 7,000 new medicines currently in development, patients today have more hope for a better quality of life.
See one biopharmaceutical researcher’s story here on the importance of persevering in the face of setbacks.
PrintOur news
-
14 March 2024
-
26 February 2024
-
NovaMedica team wishes you a Merry Christmas and a Happy New Year!
26 December 2023
Media Center
-
Putin has stated that Russia will support Africa in the struggle against epidemics
19 April 2024
-
The Russian government has expanded the list of vital and essential drugs
19 April 2024
-
Achieving FAIR bacterial identification
18 April 2024
-
Unique testing regimen could prevent nuclease contamination
18 April 2024