
Alzheimer's meds are here. But for the Down syndrome community, it's still the 1980s
09 March 2023
Thirty years ago, the Down syndrome community helped scientists uncover critical learnings about the genetic basis of Alzheimer’s disease. Now, in 2023, we stand on the cusp of new treatments reaching the market, and yet this population has never been included in the clinical research that has underpinned regulatory approvals for these medicines.
Scientists and advocates say it’s about time.
Ninety percent of people with Down syndrome, also known as trisomy 21, will develop Alzheimer’s. They are the largest population of genetically determined Alzheimer’s disease in the world, at 6 million people.
People with Down syndrome are now living to be 60 or older, but these patients “are going to face death by Alzheimer's disease with no therapeutic intervention along the way,” according to Richard Fisher, Ph.D., chief scientific officer for clinical research advocacy group LuMind IDSC, which connects the Down syndrome community with researchers.
Back in the 1980s, researchers found plaques in the autopsied brains of patients with Down syndrome that were identical to those seen in elderly patients. This led them to discover the amyloid precursor protein, which causes an overproduction of beta amyloid in the brain. The buildup of beta amyloid is a hallmark of Alzheimer’s. Tracing things back, scientists hunted for the gene that encodes for APP and found that it was chromosome 21: the exact chromosome that people with Down syndrome have an extra copy of.
If you have an extra copy of that gene, your brain overproduces amyloid, eventually leading to an early-onset form of Alzheimer’s, according to Michael Rafii, M.D., Ph.D., a professor of clinical neurology at the University of Southern California’s Keck School of Medicine. He’s also medical director of the Alzheimer’s Therapeutic Research Institute and has dedicated much of his research to the crossover between Alzheimer’s disease and Down syndrome. People with Down syndrome can have plaques begin to form as early as 12 years old, he said.
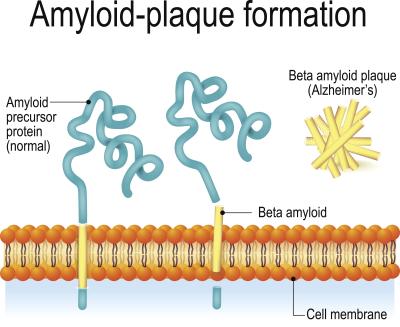
Rafii notes that research has shown that patients with Down syndrome who have partial trisomy, meaning that the chromosome is not fully duplicated, do not develop Alzheimer’s because they don’t have the triple-APP gene encoded.
“If you look at all the AD clinical trials for the past 20 years, which have brought in some of the most promising therapies, in every study the exclusion criteria says having Down syndrome, and yet people with Down syndrome are the population that taught us about APP and beta amyloid,” Rafii said. “Now is the time to bring the latest therapies to this population and not to exclude them.”
'Simply outraged'
The biopharma world has nevertheless largely stayed away from clinical research in patients with Down syndrome—with one key exception. AC Immune is currently the only prominent company working specifically with these patients. In January, the company began enrolling a cohort of people with Down syndrome in the phase 1/2 ABATE trial, which is testing the vaccine ACI-24.060. This is the second study AC Immune has conducted in this population, after a phase 1b trial. Rafii is the principal investigator for both studies.
“Disease modifying interventions are here today, and yet there's almost nothing going on to get these people into clinical trials to prove that they are good subject for that therapeutic, that it’s safe,” Fisher said. “These are very promising drug candidates … and yet there's no way to give them to somebody with Down syndrome.”
To Fisher’s knowledge, the leading treatments, Eisai and Biogen’s lecanemab, now known as Leqembi, and Eli Lilly’s donanemab have never been tested in a single patient with Down syndrome. Eisai declined to comment for this story, as did Roche’s Genentech, which also has Alzheimer’s treatments in the works.
Lilly, on the other hand, has at least tried. The company is allowing patients with Down syndrome and other common conditions into the phase 3 TRAILBLAZER-ALZ 2 study, which is due for a highly anticipated readout this year, according to a statement from Dawn Brooks, who serves as global development leader for the pharma’s three Alzheimer’s therapies: donanemab, remternetug and solanezumab.
“We have an ongoing commitment at Lilly to make our trials as representative of the broader population as possible,” she said. “While we always hope to include people with Down syndrome in our Alzheimer’s disease trials and we certainly do not take any steps to exclude them, the timing of Alzheimer’s disease onset makes identification of trial-eligible participants with Down syndrome a challenge.”
And for that reason, Lilly’s trial, which features early-onset patients, may inadvertently exclude Down syndrome patients anyway. Fisher said most trials accept patients starting in their 60s, which is the cutoff for the TRAILBLAZER-ALZ 2 trial, according to its ClinicalTrials.gov entry.
“There's no one with Down syndrome at 65 with early stage, I mean very few people. So that age cutoff needs to be fixed,” Fisher said.
So far, no patients with Down syndrome in their medical histories have been enrolled, a spokesperson for Lilly confirmed.
But the issue goes back even further. Rafii said existing treatments like Aricept, also by Eisai, have not been rigorously tested for safety or efficacy in this population, despite that medication receiving the FDA OK in 2004.
Rafii said families are split into two camps: Some don’t want to feel like a “guinea pig” and just want to enjoy the time they have together. Others are “simply outraged” that they still haven’t been included in research.
“Having seen them in clinic, a lot of families, they just want access and not to be discriminated against when it comes to access to these new therapies,” Rafii said.
That feeling of being a guinea pig is not without historical precedent, either. Like a lot of minority communities that are to this day less included in clinical research, there’s a dark history of medical experimentation in Down syndrome.
“That's all gone now, but there's the residue of mistrust there,” Fisher said. “And you can imagine if you have a child with intellectual disability, you're going to be really cautious about putting them into a clinical trial of any sort.”
AC Immune CEO Andrea Pfeifer, Ph.D., is careful in describing how important the Down syndrome community can be to overall Alzheimer’s research, calling these patients “the best model.” She calls the work her company has done with the Down syndrome community one of the “most amazing experiences” of her life. AC Immune later organized an event to discuss the science with experts, but also patients and their families. She thinks advocacy and awareness events like that, where clinicians and companies can hear from those directly affected, are key.
Awareness of the link between Alzheimer’s and Down syndrome is critically lacking. Companies and institutions are becoming involved “slowly but slowly,” according to Pfeifer.
“How many meetings did I have where [people] look at me with big eyes and say, 'Really? We didn't know that.' And they were very educated people,” the CEO said.
The same, but different
There are slight differences in the disease pathology between the genetic version of early-onset Alzheimer’s seen in Down syndrome and what the general population experiences. For Down syndrome, overproduction of beta amyloid is thought to be the problem. But in the general population, Alzheimer’s tends to occur when the brain under excretes beta amyloid. In both cases, it builds up and causes plaques and tangles in the brain, leading to cognitive decline. That part of the disease is identical, Rafii said.
“I think it's quite compelling to think about Alzheimer’s disease biomarkers in Down syndrome teaching us about AD biomarkers in general,” Rafii said.
Thanks to researchers like Rafii, plenty of data have been published on emerging biomarkers for Down syndrome that have become more understood just in the past couple of years. Advocacy groups like the Global Down Syndrome Foundation and the National Down Syndrome Society are leading the charge to get the word out.
“We're really on the leading edge of getting that data out … to researchers and pharma so they can look at the data and analyze it themselves to assess whether they want to get involved,” Rafii said. “Now that we have the clinical symptoms anchored with biomarkers, I think a lot of biotech and pharma will take notice of this population.”
Studies conducted by Rafii, AC Immune and others have, crucially, shown that patients with Down syndrome are fully capable of participating in this type of research.
“At the beginning, to be very honest, we didn't know how to do it,” Pfeifer said. AC Immune worked closely with William Mobley, M.D., Ph.D., a neurologist at University of California, San Diego, to figure out how to communicate with patients and engage them in the study. The company even organized birthday events to make the community more comfortable. Blood samples are “much more of an event” for a patient with Down syndrome, for example, so there was a lot of learning to ensure patients felt safe.
“This was simply to figure out, can it be done in Down syndrome or not? Because these are sensitive populations of people,” Pfeifer said. “And we were so amazed how compliant they were, how the families supported us. We did not lose a single patient.”
One interesting finding in that study is that the patients with Down syndrome had a higher level of anti-abeta antibodies to start with, which are antibodies that can help the brain clear amyloid plaques. AC Immune’s vaccine aims to spur this response as well, and this group of patients had a higher response to the vaccine. Pfeifer said this isn’t well understood, but she suspects that because Down syndrome patients develop plaques so early, their bodies have permanent exposure and develop a different response over time. Because of this difference in antibody levels, Pfeifer said that safety and dosage were considered very carefully for advancement to the Down syndrome population.
AC Immune’s latest study started off with a regular Alzheimer’s population to test the right dose. Now that safety has been established, they are opening up enrollment for those with Down syndrome. While the company is working on the phase 1/2 alone, Pfeifer said a future phase 3 will likely need a partner to get to commercialization.
In earlier mice studies, using models that have the extra chromosome 21, AC Immune’s vaccine seemed to improve cognition.
A key element of any clinical trial is informed consent. Patients—and in Alzheimer’s trials their caregivers—must have a study explained to them clearly to understand the risks, the potential benefit or lack of benefit and so on. Mobley and Rafii, who Pfeifer referred to as the “top guys” in this area of research, helped establish the clinical endpoints and ensure that the lengthy but critically important consent process was conducted.
Rafii points to an National Institutes of Health (NIH) working group that he chaired to address informed consent, which is part of the broader INCLUDE Initiative that is trying to get people with Down syndrome into existing trials—Alzheimer’s being one of the focus areas.
Studies have to be conducted at knowledgeable research sites, with staff trained to understand and care for these patients and their special needs, Rafii said. There’s a small number of sites out there with this kind of training and that’s thanks to people like Rafii who saw a need and created the expertise needed.
How it started, and where we're going
Rafii became interested in Down syndrome as he began to see more and more adults with the condition coming through his memory disorders clinic, leading him to open one just for this population to explore the genetic causes.
He was also working with the Alzheimer’s Disease Neuroimaging Initiative, or ADNI, which over 20 years has “taught us about the natural history of Alzheimer's disease and biomarkers,” Rafii said. All clinical trials for Alzheimer’s have taken lessons from ADNI. He realized that no such initiative existed for Down syndrome, so he kick-started the Down Syndrome Biomarker Initiative.
The pilot, sponsored by Johnson & Johnson unit Janssen Research and Development, set out to learn whether patients could get a PET scan, MRI or blood tests, because “the way that you go about assessing someone with Down syndrome is different than in the general population,” Rafii noted. They also sought to learn how to conduct cognitive testing to show the development of dementia.
“Sure enough, they can participate in such a study,” Rafii said of patients with Down syndrome. The pilot for the first time used tau PET imaging, which Rafii said was a breakthrough in overall Alzheimer’s research, allowing for visualization of the changes in patients’ brains over the course of a clinical trial. The DSBI was so successful that the NIH launched a much larger version called Alzheimer's Biomarkers Consortium – Down Syndrome, or ABC-DS, in 2020 where 600 adults will be followed until 2025.
Rafii has also worked to establish the Alzheimer's Clinical Trials Consortium – Down Syndrome, or ACTC-DS, which has sites in Europe and the U.S. that bring together the right for clinical trials.
Now, it’s time for pharma to act.
“In my opinion, the drug companies need to understand that these advocacy groups are laying the groundwork for a better understanding by the community. And now the drug companies should engage with researchers to run studies,” Rafii said.
Of course, these studies can’t be run in the exact same way as your average clinical trial. Fisher, Pfeifer and Rafii rattled off a list of caveats to their otherwise wholehearted advocacy.
One key challenge is that people with Down syndrome typically have co-morbidities, or unstable health conditions that would exclude them from a trial. The ACTC-DS has established the Trial-Ready Cohort – Down Syndrome, or TRC-DS, to prescreen potential participants to ensure they can participate.
Another hurdle is the study goals. Fisher said that most of the common assessment scales used to diagnose and treat Alzheimer’s are not sensitive enough to pick up on changes in a person with an intellectual disability, so they are being rethought but have yet to be validated in a major trial. He called this a “big, big barrier.”
The monoclonal antibody class of drugs that is leading in the clinic now has known risks of adverse events in patients with a higher amyloid load, Rafii said. Due to the genetic, early nature of Alzheimer’s in patients with Down syndrome, that means they have a higher load and thus the higher risk. It’s still unknown whether the same doses and titration can be used in patients with Down syndrome. These studies must be done “to ensure that these drugs are just as safe in the Down syndrome population,” Rafii said.
If companies are willing to work through these issues, they could find a “win-win” situation, according to Pfeifer. When AC Immune was considering how to test their Alzheimer’s vaccine, Pfeifer said the Down syndrome community was appealing because the genetic nature of their disease means the patients are all very similar. Fisher said that, save for the known comorbidities, early onset Down syndrome patients can actually be healthier than the older patients who might be enrolled for Alzheimer’s.
Rafii said a trial conducted in patients with Down syndrome could potentially be done with much fewer patients and faster. A typical placebo-controlled sporadic Alzheimer’s trial in the general population needs thousands of patients. Lilly’s trial, for instance, has 1,800 participants. But if you pick the right assessments and the right age group, patients with Down syndrome could show efficacy a lot faster given the earlier course of their disease.
Fisher believes that companies want to avoid “complexity” in registrational studies that could form the basis of a regulatory approval. But once the approvals are granted, companies could and should test their treatments in patients with Down syndrome.
“After approval, one would expect this is perfectly doable,” Fisher said. “But … the drug company has to be very motivated to do it, somehow, whether for moral, ethical reasons or somebody's pushing them to do it.”
Fisher said individuals within the pharma companies need to champion the idea of investing in research for communities like this. It’s not going to affect a huge population—meaning it won’t make the millions or billions that a company needs to get behind an idea, but it will make a major impact to the families.
“It's not a big, shiny object,” Fisher admitted.
Lilly does have those advocates in-house. The Indianapolis Big Pharma recently signed a partnership with LuMind IDSC aimed at accelerating research and awareness for conditions that affect people with Down syndrome, specifically Alzheimer’s. LuMind IDSC will provide its expertise on what needs to be done for a clinical trial with this population. The two will also create an awareness campaign to boost participation in clinical research and develop educational videos featuring patients with Down syndrome participating in brain imaging scans that are key to Alzheimer’s research.
Lilly’s Brooks said that the pharma is working with advocacy groups “to support biomarker analysis of natural progression studies, increase awareness of clinical trials of Alzheimer’s disease therapies and to prepare people with Down syndrome for the MRI and PET brain scans typically required for these trials.”
The Down syndrome community hasn’t stayed silent. As the Centers for Medicare & Medicaid Services (CMS) considered whether to provide coverage for Eisai and Biogen’s controversial medicine Aduhelm back in 2022, the community wrote as many as 3,000 letters asking for coverage or at the very least, safety studies to see if the therapy could be used at all. The original proposal had exclusion criteria for “any neurological or other medical condition (other than AD) that may significantly contribute to cognitive decline.” CMS ultimately decided against this exclusion criteria, noting the overwhelming number of comments asking for a rethink of what one letter referred to as discrimination.
Pfeifer was at least happy to see Down syndrome mentioned at all. She hopes that as more data come in for anti-abeta therapies, companies will follow.
Because there’s another key difference that is particularly cruel for the families of Down syndrome patients: The disease manifests so early that mothers and fathers act as caregivers for their children, not the other way around, as is the case with Alzheimer’s in the general population, which is defined by older children watching their parents or grandparents fade away.
Pfeifer remembers one mother at the advocacy event her company organized saying she was trying to do what is best for her child.
“The emotional aspect is enormous,” Pfeifer said. “If this would be my only achievement in life and I would generate a vaccine for this population, I would be already very happy.” She said, while noting with a laugh that she, of course, ultimately wants the “bigger thing”: a treatment for all Alzheimer’s patients.
PrintOur news
-
14 March 2024
-
26 February 2024
-
NovaMedica team wishes you a Merry Christmas and a Happy New Year!
26 December 2023
Media Center
-
Putin has stated that Russia will support Africa in the struggle against epidemics
19 April 2024
-
The Russian government has expanded the list of vital and essential drugs
19 April 2024
-
Achieving FAIR bacterial identification
18 April 2024
-
Unique testing regimen could prevent nuclease contamination
18 April 2024